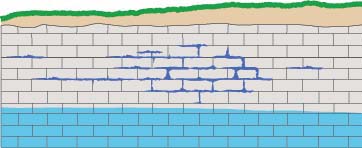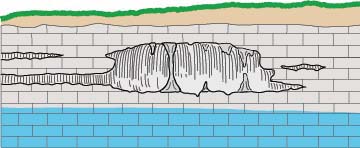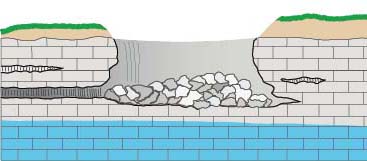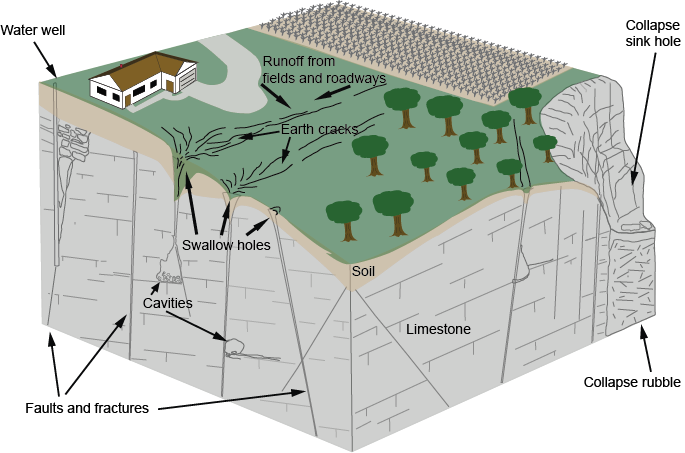
Water draining through cracks and joints in limestone bedrock creating small passages,
which can grow into caverns and sometimes collapse into sinkholes.
About Karst
The German (and now general) term “Karst” comes from the Slovene word "Kras," meaning rocky, which was given to a limestone plateau region of the Dinaric Alps in the Balkan countries of southeast Europe, and describes land generally lacking surface streams and marked by sinkholes, caves and earth cracks because water drains mainly or exclusively underground.
Typically, karst terrain forms where water has dissolved and eroded soluble bedrock, particularly carbonate rocks such as limestone and dolomite, and gypsum, by enlarging cracks into underground conduits that can enlarge into caverns and sometimes collapse to form sinkholes.

Earth cracks, such as this one at the Mystery Valley Preserve, illustrate how water works its way down through small cracks and joints in the bedrock.

The main entrance to Hendries River Water Cave on the MKC's Fiborn Preserve is a stream canyon.
Not only do underground drainage and karst formation create features such as caves that fascinate people and provide unusual habitats for often-rare plants and animals, it creates significant problems in water supply, waste disposal, construction and other land uses. Pollutants from agricultural runoff or trash dumped into sinkholes can quickly reach groundwater used for drinking, without the filtering provided by drainage through soil. Groundwater in karst areas can flow faster than 30 feet per hour, meaning pollutants can travel 10 miles in only a week.
To people familiar with the extensive limestone caves of the southeastern United States, and elsewhere, Michigan may not seem like a karst region. However Paleozoic limestone and dolomite, formed in ancient shallow seas from the shells of marine molluscs, crop out around the periphery of Michigan. These have been scoured by waves of advancing glaciers, and covered in places by deep layers of clay, sand and gravel left in the melting glaciers’ wake, but in area where glacial deposits are thin, karst features can develop.
Large sinkholes, dramatic earth cracks and disappearing lakes dot Alpena and Presque Isle counties in the northeastern lower peninsula. Limestone and dolomite outcrops of the Niagara Escarpment in the upper peninsula are marked by cracks, sinkholes, springs and a few caves. Sinkholes in Monroe County in the southeastern lower peninsula drain water that resurges from the Great Sulphur Spring in Lake Erie. Exposed gypsum karst near Grand Rapids and limestone in the northeastern lower peninsula forms the basis of large mining and quarrying industries.
Limestone, Water, and Carbon Dioxide
The ingredients for forming a karst terrain are:
- a carbonate bedrock of limestone or marble, (consisting mostly of the mineral calcite, CaCO3), or dolomitic limestones, (which also contains magnesium as MgCO3);
- water (H20) as rainfall and surface streams;
- carbon dioxide (CO2), which is present in the atmosphere at a low concentration and, especially, formed in soils by the decay of vegetation.
Limestone or dolomitic limestone form from the shells of molluscs and coral reefs accumulating in seas over vast periods of time, and being compacted into rock (or recrystallized to form marble). In Michigan this deposition occurred in the early Paleozoic era, roughly 500 million to 350 million years ago.
These carbonate rocks are strong but susceptible to corrosion by acidic water, which is formed by the reaction between water and dissolved carbon dioxide forming carbonic acid (CO2 + H2O = H2CO3). The acid reacts with limestone to dissolve it by the reaction H2CO3 + CaCO3 = Ca+2 + 2HCO3-1, the product being calcium bicarbonate as dissolved ions.
The whole process requires access points in the bedrock, provided by joints (cracks) and weaknesses between rock strata. The acidic water travels from the surface down these openings, enlarging them in the zone above the water table (the vadose zone), and then joining the flow of water in conduits that develop by the same process below the water table (the phreatic zone), forming caves. Over time these flow paths can be enlarged into voids large enough to collapse to the surface, forming sinkholes.
The whole process is complicated by various compositions and orientations of the bedrock strata, the location of joints in the rock, the locations of surface streams, and insoluble sediments such as sand and clay that can block developing conduits, and the evolution of a karst area as the land surface is lowered by erosion. It is often not obvious beforehand where water entering a sinkhole eventually returns to the surface or to a larger body of water.
In northeast and southeast Michigan, strata of another rock weakly soluble in just water, gypsum (CaSO4•2H2O), occurs in layers in the limestone. The gypsum has been more easily and quickly dissolved than limestone, by water descending joints, forming large voids that have collapsed to form sinkholes in Alpena, Presque Isle and Montmorency counties. Less prominent but similar karst features occur in Monroe County. Only small solution caves in limestone occur in these areas. That this is the process contributing to forming those karst areas is shown by the presence of large springs in Lakes Huron and Lake Erie with outflowing water saturated with dissolved gypsum. There are also gypsum bedrock exposures near Tawas City and Grand Rapids, and sinkholes are also found there. However karst features on gypsum form rapidly and are destroyed rapidly, compared to in limestone karst, due to the much great solubility of gypsum in water. The gypsum strata do not exist in the Upper Peninsula so the karst areas there are formed entirely by the solution of limestones.